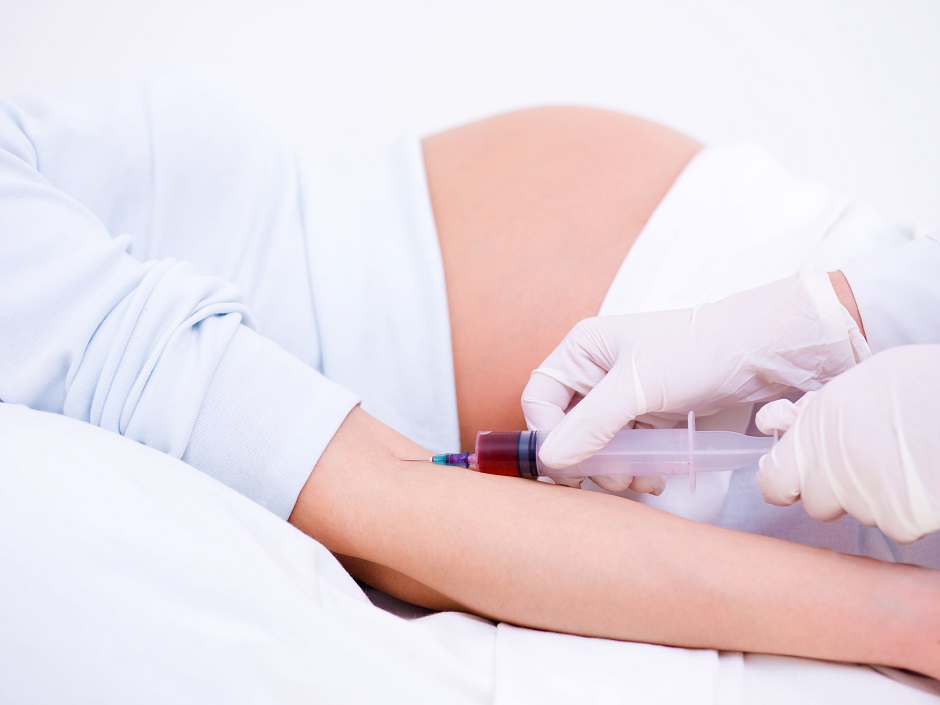
What is a RhoGAM Shot?
A RhoGAM shot refers to an injection of a medication called Rho(D) immune globulin (RhoGAM). It is administered to individuals who have Rh-negative blood type and are at risk of developing complications during pregnancy or blood transfusions.
To understand the significance of a RhoGAM shot, it’s essential to have some background knowledge about the Rh factor. The Rh factor is a protein that can be found on the surface of red blood cells. Approximately 85% of people worldwide have the Rh factor, making them Rh-positive, while the remaining 15% lack it, making them Rh-negative.
During pregnancy, problems can arise when the mother has Rh-negative blood, and the father has Rh-positive blood, resulting in an Rh incompatibility between the mother and the fetus. If the fetus inherits the Rh-positive factor from the father, the mother’s immune system may recognize the Rh-positive blood cells as foreign and produce antibodies against them. Without intervention, about 50-75% of Rh-negative mothers carrying an Rh-positive fetus will develop antibodies against the Rh factor.
This immune response can be problematic, as the antibodies can cross the placenta and attack the fetus’s red blood cells, potentially leading to a condition called hemolytic disease of the newborn (HDN). HDN can cause severe anemia, jaundice, and in severe cases, fetal hydrops or even stillbirth.
The RhoGAM shot is specifically designed to prevent the development of antibodies in Rh-negative individuals who may come into contact with Rh-positive blood. It contains a concentrated form of antibodies against the Rh factor. When the RhoGAM shot is given to an Rh-negative person, it essentially “mops up” any Rh-positive blood cells that may have entered their system, preventing the immune system from recognizing them and initiating the production of antibodies.
The RhoGAM shot is commonly administered to Rh-negative women during pregnancy to protect future pregnancies. It is typically given around the 28th week of gestation and within 72 hours after giving birth, miscarriage, ectopic pregnancy, or any event where there is a potential mixing of the mother’s and fetus’s blood. This injection ensures that the mother does not develop antibodies that could harm a future Rh-positive fetus. The standard dosage of RhoGAM is 300 micrograms (μg) of Rho(D) immune globulin.
The RhoGAM shot is highly effective in preventing the development of Rh antibodies in Rh-negative individuals, with almost 100% efficacy when administered correctly and within the recommended timeframe. RhoGAM has been in use for several decades and is considered a safe and well-tolerated medication. It is approved by regulatory authorities for use in preventing Rh sensitization.
Additionally, RhoGAM shots may be given in other situations where there is a risk of exposure to Rh-positive blood, such as after a blood transfusion involving Rh-positive blood or following certain diagnostic or therapeutic procedures, like chorionic villus sampling or amniocentesis.
What is the Rh factor?
The Rh factor refers to a protein called the Rh antigen, which is found on the surface of red blood cells. It was first discovered in Rhesus monkeys, hence the name. The presence or absence of the Rh antigen determines an individual’s Rh blood type.
The Rh system is divided into two main groups: Rh-positive (Rh+) and Rh-negative (Rh-). Approximately 85% of the population is Rh-positive, meaning they have the Rh antigen on their red blood cells. The remaining 15% are Rh-negative, lacking the Rh antigen.
In blood transfusions, the Rh factor is crucial to consider. Rh-positive blood can be given to Rh-positive and Rh-negative individuals without causing major issues. However, Rh-negative individuals should not receive Rh-positive blood, as it can trigger an immune response and lead to the production of antibodies against the Rh antigen.
During pregnancy, the Rh factor becomes particularly significant for Rh-negative women. If an Rh-negative woman conceives a child with an Rh-positive man, there is a risk of Rh incompatibility between the mother and fetus. If the fetus inherits the Rh antigen from the father, the mother’s immune system may recognize it as foreign and produce antibodies against it. These antibodies can cross the placenta and attack the fetus’s red blood cells, potentially causing hemolytic disease of the newborn (HDN) or other complications.
To prevent Rh sensitization and the associated risks, Rh-negative women are given Rho(D) immune globulin (RhoGAM) shots during pregnancy. RhoGAM contains antibodies against the Rh factor, which can prevent the mother’s immune system from producing its own antibodies. The shots are typically administered around the 28th week of pregnancy and within 72 hours after childbirth or any other situation where there may be exposure to Rh-positive blood.
In terms of statistics, it is estimated that around 15% of the global population is Rh-negative, while the remaining 85% is Rh-positive. The prevalence of Rh-negative blood varies among different ethnic groups and populations. For example, the highest proportion of Rh-negative individuals is found in people of European descent, with approximately 16% to 18% being Rh-negative. In contrast, the incidence of Rh-negative blood is lower in populations such as Africans and Asians.
Overall, understanding the Rh factor is crucial in blood transfusions, prenatal care, and managing potential risks during pregnancy to ensure the health and well-being of individuals with different Rh blood types.
What is Rh incompatibility?
Rh incompatibility, also known as Rh isoimmunization or Rh disease, is a condition that can occur during pregnancy when there is an incompatibility between the Rh blood types of the mother and the fetus. It specifically refers to the situation where the mother is Rh-negative (lacks the Rh factor) and the fetus is Rh-positive (has the Rh factor).
Rh incompatibility affects a significant number of pregnancies worldwide. Approximately 15% of the population is Rh-negative, and if an Rh-negative woman carries an Rh-positive fetus, there is a risk of sensitization and the development of Rh antibodies.
Sensitization occurs when the mother’s immune system recognizes the Rh-positive red blood cells from the fetus as foreign and produces antibodies against them. It usually happens during childbirth or when there is a mixing of the mother’s and fetus’s blood, such as during miscarriage, ectopic pregnancy, or invasive prenatal procedures.
If the mother becomes sensitized and develops Rh antibodies, subsequent pregnancies with Rh-positive fetuses are at risk of complications. The antibodies can cross the placenta and enter the fetal bloodstream, leading to the destruction of the fetus’s red blood cells. This can result in hemolytic disease of the newborn (HDN) or erythroblastosis fetalis.
HDN can range in severity, depending on the amount of fetal red blood cell destruction. In mild cases, the fetus may experience minimal harm, while in severe cases, it can lead to life-threatening complications. HDN can cause anemia, jaundice, enlarged liver and spleen, and edema in the fetus.
Fortunately, medical interventions have greatly improved the management and prevention of Rh incompatibility complications. The introduction of Rho(D) immune globulin (RhoGAM) shots has been a significant advancement. Rh-negative women are typically administered RhoGAM during pregnancy around 28 weeks gestation and within 72 hours after childbirth or any event that may lead to the mixing of the mother’s and fetus’s blood. RhoGAM works by preventing sensitization, suppressing the mother’s immune response against the Rh-positive blood cells.
Regular monitoring of the fetus’s well-being is crucial in managing Rh incompatibility. This includes frequent check-ups, blood tests, and ultrasound examinations to assess the fetal blood count, detect signs of anemia or other complications, and ensure appropriate medical interventions if needed.
Thanks to these interventions, the incidence of severe HDN has significantly decreased in developed countries. However, it’s essential for healthcare providers to remain vigilant in identifying and managing Rh incompatibility to ensure the best outcomes for both the mother and the baby.
How is RhoGAM injected?
RhoGAM is typically administered as an intramuscular injection, meaning it is injected into a muscle. The most common site for the injection is the deltoid muscle, which is the muscle located at the upper arm, just below the shoulder.
Here is a step-by-step explanation of how a RhoGAM shot is typically administered:
Preparation: The healthcare professional will gather the necessary supplies, including a vial or syringe containing RhoGAM, alcohol swabs, and a sterile needle.
Patient position: The individual receiving the injection will usually be asked to sit or lie down in a comfortable position, exposing the upper arm where the injection will be given.
Cleansing the injection site: The healthcare professional will clean the injection site, typically the deltoid muscle, using an alcohol swab. This step ensures that the area is free from any potential contaminants.
Needle insertion: The healthcare professional will use a sterile needle to administer the RhoGAM injection. They will swiftly insert the needle into the muscle at a 90-degree angle. This process is usually quick and may cause a brief moment of discomfort.
Injection of RhoGAM: Once the needle is correctly positioned in the muscle, the healthcare professional will slowly inject the RhoGAM medication. The speed of injection may vary, but it is generally administered in a controlled manner.
Needle removal: After the RhoGAM is completely injected, the healthcare professional will withdraw the needle from the muscle.
Post-injection care: A cotton swab or sterile bandage may be applied to the injection site to prevent bleeding. The individual may be advised to gently apply pressure to the site to aid in the formation of a clot and reduce the risk of bruising.
It is important to note that the dosage and administration of RhoGAM may vary depending on the specific circumstances and the healthcare provider’s instructions. The healthcare provider will determine the appropriate dosage and injection site based on factors such as the patient’s Rh factor and the reason for administering RhoGAM.
RhoGAM is primarily used to prevent Rh sensitization, a condition in which a woman’s immune system produces antibodies that can harm her unborn baby. It is commonly administered to Rh-negative women during pregnancy and after childbirth if the baby is Rh-positive. By injecting RhoGAM, the medication helps prevent the mother’s immune system from producing Rh antibodies, thereby protecting future pregnancies.
When do pregnant women get the RhoGAM shot?
Pregnant women who are Rh-negative typically receive RhoGAM shots at specific times during their pregnancy to prevent sensitization to the Rh factor and minimize the risk of complications. The timing of RhoGAM administration is typically determined based on established guidelines and the individual’s specific circumstances. Here are the common situations in which RhoGAM shots are given to pregnant women:
- Around 28 Weeks of Gestation: The first RhoGAM shot is often administered around the 28th week of pregnancy. This timing is intended to provide protection against potential sensitization that may occur during the latter stages of pregnancy. Approximately 85% of Rh-negative women who receive this shot at 28 weeks gestation avoid sensitization.
- Within 72 Hours After Birth: If the baby is confirmed to be Rh-positive, the mother will receive another RhoGAM shot within 72 hours after giving birth. This shot is important because there can be a mixing of the mother’s and baby’s blood during delivery, which may trigger the production of Rh antibodies in the mother. Administering RhoGAM promptly after birth helps prevent the development of these antibodies and protects future pregnancies. It is estimated that RhoGAM given after birth reduces the risk of sensitization by over 99%.
- After Potential Exposure to Rh-Positive Blood: Pregnant women may also receive RhoGAM if they experience any events that could lead to exposure to Rh-positive blood. These situations include miscarriage, ectopic pregnancy, amniocentesis, chorionic villus sampling, trauma, abdominal trauma during pregnancy, or any other circumstances where there is a possibility of fetal-maternal bleeding. In such cases, RhoGAM is typically given within 72 hours of the event to prevent sensitization.
It’s important to note that the specific timing and dosage of RhoGAM may vary based on individual factors and medical recommendations. Healthcare providers will evaluate the circumstances of each pregnancy and provide personalized guidance on when RhoGAM should be administered.
RhoGAM has been widely used for several decades and has proven to be effective in preventing Rh sensitization. The development and use of RhoGAM have significantly reduced the incidence of Rh disease and its associated complications in newborns. It is estimated that without RhoGAM, approximately 12% of pregnancies with an Rh-negative mother and an Rh-positive father would result in Rh disease, whereas with RhoGAM, the risk is reduced to less than 1%.
Side Effects of RhoGAM shot
The RhoGAM shot (Rho(D) immune globulin) is generally considered safe and well-tolerated. Most individuals experience minimal or no side effects. However, like any medical intervention, there is a possibility of side effects, although they are typically rare. Here are some potential side effects associated with RhoGAM:
- Injection Site Reactions: The most common side effect is mild soreness, redness, or swelling at the injection site. These reactions are usually temporary and resolve on their own without any specific treatment. According to clinical studies, injection site reactions occur in about 1-3% of individuals who receive the RhoGAM shot.
- Allergic Reactions: Although rare, some individuals may have an allergic reaction to RhoGAM. Signs of an allergic reaction can include hives, itching, difficulty breathing, chest tightness, or swelling of the face, lips, tongue, or throat. Immediate medical attention should be sought if any of these symptoms occur after receiving a RhoGAM shot. The incidence of severe allergic reactions to RhoGAM is extremely low, estimated to be less than 0.1%.
- Fever or Flu-like Symptoms: In some cases, individuals may experience a low-grade fever, body aches, or flu-like symptoms following the RhoGAM injection. These symptoms are generally mild and resolve without specific treatment. The occurrence of these symptoms varies, but they are reported in less than 1% of patients who receive RhoGAM.
- Headache or Dizziness: Some individuals may experience mild headache or dizziness after receiving the RhoGAM shot. These symptoms are usually temporary and resolve on their own. The incidence of these side effects is not well-documented but is generally considered to be low.
It is important to remember that the occurrence of side effects varies from person to person, and the majority of individuals tolerate RhoGAM well without any significant issues. Healthcare providers will assess the benefits and potential risks of administering RhoGAM in each specific case and make individualized recommendations.
When not to get RhoGAM shot
While RhoGAM (Rho(D) immune globulin) is generally considered safe and beneficial in preventing Rh sensitization, there are certain situations in which the administration of RhoGAM may not be recommended or necessary. Here are some instances when a RhoGAM shot may not be given:
- Rh-Negative Individuals: RhoGAM is specifically intended for individuals who are Rh-negative. About 15% of the population is Rh-negative, while the remaining 85% is Rh-positive. Rh-positive individuals do not require RhoGAM because they naturally possess the Rh factor.
- Already Sensitized: RhoGAM is not effective in individuals who have already developed Rh antibodies or are sensitized to the Rh factor. Once sensitization has occurred, RhoGAM does not reverse or eliminate the antibodies already present in the bloodstream. Sensitization can occur due to previous pregnancies, blood transfusions, or other factors.
- Rh-Negative Fathers: RhoGAM is not administered to Rh-negative fathers. The risk of Rh sensitization only arises in Rh-negative individuals carrying an Rh-positive fetus. In cases where both parents are Rh-negative, there is no risk of Rh incompatibility, and RhoGAM is unnecessary.
- Blood Compatibility: RhoGAM is designed to prevent sensitization during situations involving the mixing of Rh-positive and Rh-negative blood. If blood transfusions are required, blood compatibility is carefully assessed, and RhoGAM may not be necessary if the transfused blood is Rh-negative or compatible with the recipient’s blood type.
It’s important to note that these situations are general guidelines, and medical decisions regarding the administration of RhoGAM should be made on an individual basis. Healthcare providers consider various factors, including blood type compatibility, medical history, and specific circumstances, when determining whether RhoGAM is necessary and appropriate.
Rh sensitization occurs when an Rh-negative individual is exposed to Rh-positive blood, leading to the development of Rh antibodies. RhoGAM, containing Rh(D) immune globulin, helps prevent sensitization by blocking the immune response to Rh-positive blood. It is typically administered to Rh-negative pregnant women around 28 weeks of gestation and within 72 hours after delivery or any other event that may lead to the mixing of Rh-positive and Rh-negative blood. RhoGAM can also be used in cases of miscarriage, ectopic pregnancy, amniocentesis, chorionic villus sampling, or trauma during pregnancy. The appropriate administration of RhoGAM helps reduce the risk of Rh sensitization and subsequent complications in future pregnancies.